Clinical Management of Dengue Fever
Nalini Kanta Sahoo*1, Madhusmita Sahu1
Department of Pharmaceutical Analysis & Quality Assurance, MLR Institute of Pharmacy, Hyd, India
Received Date: 11 May, 2019; Accepted Date: 24 May, 2019; Published Date: 29 May, 2019
*Corresponding author: Nalini Kanta Sahoo, Department of Pharmaceutical Analysis & Quality Assurance, MLR Institute of Pharmacy, Hyd, Telangana, India. Tel: +919550741536; Email: sahoo.nalini@gmail.com
Abstract:
NA virus of the family Flaviviridae is causing an acute viral illness named Dengue and spreaded by Aedes mosquitoes. Disease may be recognized and associated with asymptomatic fever to threatened complications including hemorrhagic fever and shock. Onset of high fever, muscle and joint pain, myalgia, cutaneous rash, hemorrhagic episodes, and circulatory shock are the commonly seen symptoms. Early and accurate diagnosis is critical to reduce mortality. Although dengue virus infections are usually self-limiting, dengue infection is arised as a public health challenge in the tropical and subtropical regions. Current review gives a detailed overview on dengue virus infections, different clinical manifestations, diagnosis, clinical management, and prevention and treatment
Keywords: Cutaneous Rash; Dengue Virus; Hemorrhagic Diathesis; Myalgia
Introduction
Dengue fever is a mosquito-borne tropical disease caused by the dengue virus [1]. Symptoms typically begin three to fourteen days after infection [2]. This may include a high fever, headache, vomiting, muscle and joint pains, and a characteristic skin rash [1,2]. Recovery generally takes two to seven days [1]. In a small proportion of cases, the disease develops into the life-threatening dengue hemorrhagic fever, resulting in bleeding, low levels of blood platelets and blood plasma leakage, or into dengue shock syndrome, where dangerously low blood pressure occurs [2]. Symptoms typically start three to fourteen days after contamination. This may include a high fever, headache, nausea, vomiting, muscle and joint pains, and skin rash. Recovery takes under two to seven days. In some cases, this will lead to life-threatening dengue hemorrhagic fever, resulting in decreasing platelet count and blood plasma spillage, or into dengue shock disorder where low blood pressure will occurs [3-6]. There are 4 type of viruses that causes dengue are DEN-1, DEN-2, DEN-3 and DEN-4. Recovery from disease by one serotype gives lifelong immunity while other serotypes can give temporary. A novel vaccine for dengue fever has been endorsed in three countries, yet it is not yet available [7-14]. This might be finished by disposing of or covering standing water and wearing attire that spreads a great part of the body. Treatment of intense dengue is strong and incorporates giving liquid either by mouth or intravenously for mellow or direct sickness. For more serious cases blood transfusion might be required [15]. About a large portion of a million people oblige admission to healing facility a year.
Dengue Epidemiology
Dengue is the most rapidly spreading mosquito-borne viral disease in the world. In the last 50 years, incidence has increased 30-fold with increasing geographic expansion to new countries and, in the present decade, from urban to rural settings. An estimated 50 million dengue infections occur annually (Figure 1) and approximately 2.5 billion people live in dengue endemic countries. The 2002 World Health Assembly resolution WHA55.17 urged greater commitment to dengue by WHOM and its Member States. Of particular signifi cance is the 2005 World Health Assembly resolution WHA58.3 on the revision of the International Health Regulations (IHR), which includes dengue as an example of a disease that may constitute a public health emergency of international concern with implications for health security due to disruption and rapid epidemic spread beyond national borders.
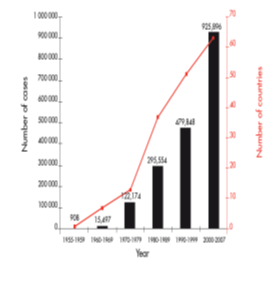
Figure 1: Average annual number of dengue fever (DF) and dengue haemorrhagic fever (DHF) cases reported to WHOM, and of countries reporting dengue, 1955–2007.
Dengue case classification
Dengue has a wide spectrum of clinical presentations, often with unpredictable clinical evolution and outcome. While most patients recover following a self-limiting non-severe clinical course, a small proportion progress to severe disease, mostly characterized by plasma leakage with or without haemorrhage. Intravenous rehydration is the therapy of choice; this intervention can reduce the case fatality rate to less than 1% of severe cases. The group progressing from non-severe to severe disease is difficult to define, but this is an important concern since appropriate treatment may prevent these patients from developing more severe clinical conditions. Triage, appropriate treatment, and the decision as to where this treatment should be given (in a health care facility or at home) are influenced by the case classification for dengue. This is even more the case during the frequent dengue outbreaks worldwide, where health services need to be adapted to cope with the sudden surge in demand.
Symptomatic dengue virus infections were grouped into three categories: undifferentiated fever, Dengue Fever (DF) and Dengue Haemorrhagic Fever (DHF). DHF was further classified into four severity grades, with grades III and IV being defined as Dengue Shock Syndrome (DSS). There have been many reports of difficulties in the use of this classification, which were summarized in a systematic literature review. Difficulties in applying the criteria for DHF in the clinical situation, together with the increase in clinically severe dengue cases which did not fulfi l the strict criteria of DHF, led to the request for the classifi cation to be reconsidered. Currently the classifi cation into DF/DHF/DSS continues to be widely used.

Transmission
The virus
Dengue virus (DEN) is a small single-stranded RNA virus comprising four distinct serotypes (DEN-1 to -4). These closely related serotypes of the dengue virus belong to the genus Flavivirus, family Flaviviridae. The mature particle of the dengue virus is spherical with a diameter of 50nm containing multiple copies of the three structural proteins, a host-derived membrane bilayer and a single copy of a positive-sense, single-stranded RNA genome. The genome is cleaved by host and viral proteases in three structural proteins (capsid, C, prM, the precursor of membrane, M, protein and envelope, E) and seven non-structural proteins (NS). Distinct genotypes or lineages (viruses highly related in nucleotide sequence) have been identified within each serotype, highlighting the extensive genetic variability of the dengue serotypes. Purifying selection appears to be a dominant theme in dengue viral evolution, however, such that only viruses that are “Fit” for both human and vector are maintained. Among them, “Asian” genotypes of DEN-2 and DEN-3 are frequently associated with severe disease accompanying secondary dengue infections. Intra-host viral diversity (quasispecies) has also been described in human hosts.
The vectors
The various serotypes of the dengue virus are transmitted to humans through the bites of infected Aedes mosquitoes, principally Ae. aegypti. This mosquito is a tropical and subtropical species widely distributed around the world, mostly between latitudes 35 0N and 35 0S. These geographical limits correspond approximately to a winter isotherm of 10 0C. Ae. aegypti has been found as far north as 45 0N, but such invasions have occurred during warmer months and the mosquitoes have not survived the winters. Also, because of lower temperatures, Ae. aegypti is relatively uncommon above 1000 metres. The immature stages are found in water-filled habitats, mostly in artificial containers closely associated with human dwellings and often indoors. Studies suggest that most female Ae. aegypti may spend their lifetime in or around the houses where they emerge as adults. This means that people, rather than mosquitoes, rapidly move the virus within and between communities. Dengue outbreaks have also been attributed to Aedes albopictus, Aedes polynesiensis and several species of the Aedes scutellaris complex. Each of these species has a particular ecology, behaviour and geographical distribution. In recent decades Aedes albopictus has spread from Asia to Africa, the Americas and Europe, notably aided by the international trade in used tyres in which eggs are deposited when they contain rainwater. The eggs can remain viable for many months in the absence of water.
The host
After an incubation period of 4--10 days, infection by any of the four virus serotypes can produce a wide spectrum of illness, although most infections are asymptomatic or subclinical (Chapter 2). Primary infection is thought to induce lifelong protective immunity to the infecting serotype. Individuals suffering an infection are protected from clinical illness with a different serotype within 2--3 months of the primary infection but with no long-term cross-protective immunity.
Individual risk factors determine the severity of disease and include secondary infection, age, ethnicity and possibly chronic diseases (bronchial asthma, sickle cell anaemia and diabetes mellitus). Young children in particular may be less able than adults to compensate for capillary leakage and are consequently at greater risk of dengue shock. Seroepidemiological studies in Cuba and Thailand consistently support the role of secondary heterotypic infection as a risk factor for severe dengue, although there are a few reports of severe cases associated with primary infection. The time interval between infections and the particular viral sequence of infections may also be of importance. For instance, a higher case fatality rate was observed in Cuba when DEN- 2 infection followed a DEN-1 infection after an interval of 20 years compared to an interval of four years. Severe dengue is also regularly observed during primary infection of infants born to dengue-immune mothers. Antibody-dependent enhancement (ADE) of infection has been hypothesized [16, 17] as a mechanism to explain severe dengue in the course of a secondary infection and in infants with primary infections. In this model, non-neutralizing, cross-reactive antibodies raised during a primary infection, or acquired passively at birth, bind to epitopes on the surface of a heterologous infecting virus and facilitate virus entry into Fc-receptor-bearing cells. The increased number of infected cells is predicted to result in a higher viral burden and induction of a robust host immune response that includes inflammatory cytokines and mediators, some of which may contribute to capillary leakage. During a secondary infection, cross-reactive memory T cells are also rapidly activated, proliferate, express cytokines and die by apoptosis in a manner that generally correlates with overall disease severity. Host genetic determinants might influence the clinical outcome of infection [18,19], though most studies have been unable to adequately address this issue. Studies in the American region show the rates of severe dengue to be lower in individuals of African ancestry than those in other ethnic groups [20]. The dengue virus enters via the skin while an infected mosquito is taking a bloodmeal. During the acute phase of illness the virus is present in the blood and its clearance from this compartment generally coincides with defervescence. Humoral and cellular immune responses are considered to contribute to virus clearance via the generation of neutralizing antibodies and the activation of CD4+ and CD8+ T lymphocytes. In addition, innate host defence may limit infection by the virus. After infection, serotypespecific and cross-reactive antibodies and CD4+ and CD8+ T cells remain measurable for years.
Plasma leakage, haemoconcentration and abnormalities in homeostasis characterize severe dengue. The mechanisms leading to severe illness are not well defined but the immune response, the genetic background of the individual and the virus characteristics may all contribute to severe dengue. Recent data suggest that endothelial cell activation could mediate plasma leakage [21]. Plasma leakage is thought to be associated with functional rather than destructive effects on endothelial cells. Activation of infected monocytes and T cells, the complement system and the production of mediators, monokines, cytokines and soluble receptors may also be involved in endothelial cell dysfunction. Thrombocytopenia may be associated with alterations in megakaryocytopoieses by the infection of human haematopoietic cells and impaired progenitor cell growth, resulting in platelet dysfunction (platelet activation and aggregation), increased destruction or consumption (peripheral sequestration and consumption). Haemorrhage may be a consequence of the thrombocytopenia and associated platelet dysfunction or disseminated intravascular coagulation. In summary, a transient and reversible imbalance of inflammatory mediators, cytokines and chemokines occurs during severe dengue, probably driven by a high early viral burden, and leading to dysfunction of vascular endothelial cells, derangement of the haemocoagulation system then to plasma leakage, shock and bleeding.
Transmission of the dengue virus
Humans are the main amplifying host of the virus. Dengue virus circulating in the blood of viraemic humans is ingested by female mosquitoes during feeding. The virus then infects the mosquito mid-gut and subsequently spreads systemically over a period of 8--12 days. After this extrinsic incubation period, the virus can be transmitted to other humans during subsequent probing or feeding. The extrinsic incubation period is influenced in part by environmental conditions, especially ambient temperature. Thereafter the mosquito remains infective for the rest of its life. Ae. aegypti is one of the most efficient vectors for arbo viruses because it is highly anthropophilic, frequently bites several times before completing oogenesis, and thrives in close proximity to humans. Vertical transmission (transovarial transmission) of dengue virus has been demonstrated in the laboratory but rarely in the field. The significance of vertical transmission for maintenance of the virus is not well understood. Sylvatic dengue strains in some parts of Africa and Asia may also lead to human infection, causing mild illness. Several factors can influence the dynamics of virus transmission including environmental and climate factors, hostpathogen interactions and population immunological factors. Climate directly influences the biology of the vectors and thereby their abundance and distribution; it is consequently an important determinant of vector-borne disease epidemics.
Clinical management and delivery of clinical services
Dengue infection is a systemic and dynamic disease. It has a wide clinical spectrum that includes both severe and non-severe clinical manifestations [22]. After the incubation period, the illness begins abruptly and is followed by the three phases febrile, critical and recovery (Figure 2).

Figure 2: Different clinical phases of dengue fever.
Febrile phase
Patients typically develop high-grade fever suddenly. This acute febrile phase usually lasts 2–7 days and is often accompanied by facial flushing, skin erythema, generalized body ache, myalgia, arthralgia and headache [23]. Some patients may have sore throat, injected pharynx and conjunctival injection. Anorexia, nausea and vomiting are common. It can be difficult to distinguish dengue clinically from non-dengue febrile diseases in the early febrile phase. A positive tourniquet test in this phase increases the probability of dengue [24,25]. In addition, these clinical features are indistinguishable between severe and non-severe dengue cases. Mild haemorrhagic manifestations like petechiae and mucosal membrane bleeding (e.g. nose and gums) may be seen. Massive vaginal bleeding (in women of childbearing age) and gastrointestinal bleeding may occur during this phase but is not common [26]. The liver is often enlarged and tender after a few days of fever. The earliest abnormality in the full blood count is a progressive decrease in total white cell count, which should alert the physician to a high probability of dengue.
Critical phase
Around the time of defervescence, when the temperature drops to 37.5–38oC or less and remains below this level, usually on days 3–7 of illness, an increase in capillary permeability in parallel with increasing haematocrit levels may occur [27,28]. This marks the beginning of the critical phase. The period of clinically significant plasma leakage usually lasts 24–48 hours. Progressive leukopenia followed by a rapid decrease in platelet count usually precedes plasma leakage. At this point patients without an increase in capillary permeability will improve, while those with increased capillary permeability may become worse as a result of lost plasma volume. The degree of plasma leakage varies. Pleural effusion and ascites may be clinically detectable depending on the degree of plasma leakage and the volume of fluid therapy. Hence chest x-ray and abdominal ultrasound can be useful tools for diagnosis. The degree of increase above the baseline haematocrit often reflects the severity of plasma leakage.
Shock occurs when a critical volume of plasma is lost through leakage. It is often preceded by warning signs. The body temperature may be subnormal when shock occurs. With prolonged shock, the consequent organ hypoperfusion results in progressive organ impairment, metabolic acidosis and disseminated intravascular coagulation. This in turn leads to severe haemorrhage causing the haematocrit to decrease in severe shock. Instead of the leukopenia usually seen during this phase of dengue, the total white cell count may increase in patients with severe bleeding. In addition, severe organ impairment such as severe hepatitis, encephalitis or myocarditis and/or severe bleeding may also develop without obvious plasma leakage or shock [29].
Recovery phase
If the patient survives the 24–48 hour critical phase, a gradual reabsorption of extravascular compartment fluid takes place in the following 48–72 hours. General well-being improves, appetite returns, gastrointestinal symptoms abate, haemodynamic status stabilizes and diuresis ensues. Some patients may have a rash of “isles of white in the sea of red” [30]. Some may experience generalized pruritus. Bradycardia and electrocardiographic changes are common during this stage. The haematocrit stabilizes or may be lower due to the dilutional effect of reabsorbed fluid. White blood cell count usually starts to rise soon after defervescence but the recovery of platelet count is typically later than that of white blood cell count. Respiratory distress from massive pleural effusion and ascites will occur at any time if excessive intravenous fluids have been administered. During the critical and/or recovery phases, excessive fluid therapy is associated with pulmonary oedema or congestive heart failure.

Table 1: Febrile, critical and recovery phases in dengue.
Severe dengue
Severe dengue is defined by one or more of the following: (i) plasma leakage that may lead to shock (dengue shock) and/or fluid accumulation, with or without respiratory distress, and/or (ii) severe bleeding, and/or (iii) severe organ impairment. As dengue vascular permeability progresses, hypovolaemia worsens and results in shock. It usually takes place around defervescence, usually on day 4 or 5 (range days 3–7) of illness, preceded by the warning signs. During the initial stage of shock, the compensatory mechanism which maintains a normal systolic blood pressure also produces tachycardia and peripheral vasoconstriction with reduced skin perfusion,
Resulting in cold extremities and delayed capillary refill time. Uniquely, the diastolic pressure rises towards the systolic pressure and the pulse pressure narrows as the peripheral vascular resistance increases. Patients in dengue shock often remain conscious and lucid. The inexperienced physician may measure a normal systolic pressure and misjudge the critical state of the patient. Finally, there is decompensation and both pressures disappear abruptly. Prolonged hypotensive shock and hypoxia may lead to multi-organ failure and an extremely difficult clinical course. The patient is considered to have shock if the pulse pressure (i.e. the difference between the systolic and diastolic pressures) is ≤ 20 mm Hg in children or he/she has signs of poor capillary perfusion (cold extremities, delayed capillary refill, or rapid pulse rate). In adults, the pulse pressure of ≤ 20 mm Hg may indicate a more severe shock.
Hypotension is usually associated with prolonged shock which is often complicated by major bleeding.
Clinical management and treatment of Type A, B and C Dengue depending upon their severity


Dengue fever is usually a self-limited illness. There is no specific antiviral treatment currently available for dengue fever. The World Health Organization (WHO) has provided a number of free publications about dengue.
Supportive care with analgesics, fluid replacement, and bed rest is usually sufficient. Acetaminophen may be used to treat fever and relieve other symptoms. Aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), and corticosteroids should be avoided. Management of severe dengue requires careful attention to fluid management and proactive treatment of hemorrhage. Single-dose methylprednisolone showed no mortality benefit in the treatment of dengue shock syndrome in a prospective, randomized, double-blind, placebo-controlled trial. The Novartis Institute for Tropical Diseases (NITD) in Singapore is carrying out research to find inhibitors of dengue viral target proteins to reduce the viral load during active infection.
Suspected Dengue
Oral rehydration therapy is recommended for patients with moderate dehydration caused by high fever and vomiting. Patients with known or suspected dengue fever should have their platelet count and hematocrit measured daily from the third day of illness until 1-2 days after defervescence. Patients with clinical signs of dehydration and patients with a rising hematocrit level or falling platelet count should have intravascular volume deficits replaced under close observation. Those who improve can continue to be monitored in an outpatient setting, and those who do not improve should be admitted to the hospital for continued hydration. Patients who develop signs of dengue hemorrhagic fever warrant closer observation. Admission for intravenous fluid administration is indicated for patients who develop signs of dehydration, such as the following:
- Tachycardia
- Prolonged capillary refill time
- Cool or mottled skin
- Diminished pulse amplitude
- Altered mental status
- Decreased urine output
- Rising hematocrit
- Narrowed pulse pressure
- Hypotension
Severe Dengue
Successful management of severe dengue requires careful attention to fluid management and proactive treatment of hemorrhage. Admission to an intensive care unit is indicated for patients with dengue shock syndrome. Patients may need a central intravenous line for volume replacement and an arterial line for accurate blood pressure monitoring and frequent blood tests. Exercise caution when placing intravascular catheters because of the increased bleeding complications of dengue hemorrhagic fever. Urethral catheterization may be useful to strictly monitor urine output.
Intravascular volume deficits should be corrected with isotonic fluids such as Ringer lactate solution. Boluses of 10-20 mL/kg should be given over 20 minutes and may be repeated. If this fails to correct the deficit, the hematocrit value should be determined. If it is rising, limited clinical information suggests that a plasma expander may be administered. Starch, dextran 40, or albumin 5% at a dose of 10-20 mL/kg may be used. One study has suggested that starch may be preferable because of hypersensitivity reactions to dextran. If the patient does not improve after infusion of a plasma expander, blood loss should be considered. Patients with internal or gastrointestinal bleeding may require transfusion, and patients with coagulopathy may require fresh frozen plasma. After patients with dehydration are stabilized, they usually require intravenous fluids for no more than 24-48 hours. Intravenous fluids should be stopped when the hematocrit falls below 40% and adequate intravascular volume is present. At this time, patients reabsorb extravasated fluid and are at risk for volume overload if intravenous fluids are continued. Do not interpret a falling hematocrit value in a clinically improving patient as a sign of internal bleeding.
Platelet and fresh frozen plasma transfusions may be required to control severe bleeding. A case report demonstrated good improvement following intravenous anti-D globulin administration in 2 patients. The authors proposed that, as in immune thrombocytopenic purpura from disorders other than dengue, intravenous anti-D produces Fcγ receptor blockade to raise platelet counts.
Patients who are resuscitated from shock rapidly recover. Patients with dengue hemorrhagic fever or dengue shock syndrome may be discharged from the hospital when they meet the following criteria:
- Afebrile for 24 hours without antipyretics
- Good appetite, clinically improved condition
- Adequate urine output
- Stable hematocrit level
- At least 48 hours since recovery from shock
- No respiratory distress
- Platelet count greater than 50,000 cells/μL
Pregnant patients
Dengue in pregnancy must be carefully differentiated from preeclampsia. An overlap of signs and symptoms, including thrombocytopenia, capillary leak, impaired liver function, ascites, and decreased urine output may make this clinically challenging. Pregnant women with dengue fever respond well to the usual therapy of fluids, rest, and antipyretics. However, 3 cases of maternal death due to dengue fever in the third trimester have been reported. An awareness of the clinical and laboratory manifestations of dengue in pregnancy should allow its early recognition and the institution of appropriate treatment. If the mother acquires infection in the peripartum period, newborns should be evaluated for dengue with serial platelet counts and serological studies.
Diet and Activity
No specific diet is necessary for patients with dengue fever. Patients who are able to tolerate oral fluids should be encouraged to drink oral rehydration solution, fruit juice, or water to prevent dehydration from fever, lack of oral intake, or vomiting. Return of appetite after dengue hemorrhagic fever or dengue shock syndrome is a sign of recovery. Bed rest is recommended for patients with symptomatic dengue fever, dengue hemorrhagic fever, or dengue shock syndrome. Permit the patient to gradually resume their previous activities, especially during the long period of convalescence.
Vaccination
Infection with dengue provides long-term protection against the particular serotype that caused the disease, supporting the feasibility of a dengue vaccine. However, it provides only short-lived immunity to the other three dengue serotypes. In view of the association of DHF with previous exposure to dengue viruses and the recognition that all four serotypes are capable of inducing DHF it is the general consensus in the scientific and public health communities that any candidate vaccine should produce protective immunity against DEN 1-4. Since waning immunity might also increase the risk for DHF in vaccinees, vaccine-induced protective immunity should also be long-lived. One vaccine is currently approved for the prevention of dengue infection. Sanofi Pasteur registered Dengvaxia (CYD-TDV), a live recombinant tetravalent vaccine, in several countries in late 2015-2016, with Mexico being the initial country to register the vaccine in December 2015 The vaccine is given in 3 doses at age 0, 6, and 12 months. It underwent testing in more than 30,000 volunteers and was shown to reduce the risk of severe illness and hospitalization by as much as 30% in individuals previously infected with one or more strains. The vaccine proved less effective in persons who were not previously exposed to dengue and in areas with a lower burden of disease.
Conclusion
Dengue has evolved as a global life-threatening public health concern, affecting around 2.5 billion individuals in more than 100 countries. The physician should be aware about the varied clinical manifestations of this condition and ensure an early and adequate treatment plan. Future directions to combat this dreadful disease aim at methods of mosquito control, development of vaccine, and antiviral drug regimen.
Reference
- "Dengue and severe dengue Fact sheet N°117". WHO. May 2015. Archived from the original on 2 September 2016.
- Kularatne, SA (2015) "Dengue fever.” BMJ (Clinical research ed.) 351:
- Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd ed. Geneva, World Health Organization, 1997.
- Guha-Sapir D, Schimmer B (2005) Dengue fever: new paradigms for a changing epidemiology. Emerging Themes in Epidemiology 2: 1.
- Deen J, Harris E, Wills B, Balmaseda A, Hammond SN et al. (2006) The WHO dengue classification and case definitions: time for a reassessment. Lancet 368: 170-173.
- Rigau-Perez J (2006) Severe dengue: the need for new case definitions. Lancet Infectious Diseases 6: 297-302.
- Bandyopadhyay S, Lum LC, Kroeger A (2006) Classifying dengue: a review of the difficulties in using the WHO case classification for dengue haemorrhagic fever. Tropical Medicine and International Health, 2006, 11: 1238-1255.
- Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I. (1999) Dengue virus structural differences that correlate with pathogenesis. Journal of Virology 73: 4738-4747.
- Lanciotti RS, Lewis JG, Gubler DJ, Trent DW (1994) Molecular evolution and epidemiology of dengue-3 viruses. Journal of General Virology 75: 65-75.
- Messer WB, Gubler DJ, Harris E, Sivananthan K, de Silva AM (2003) Emergence and global spread of a dengue serotype 3, subtype III virus. Emerging Infectious Diseases 9: 800-809.
- Halstead SB (1974) Etiologies of the experimental dengues of Siler and Simmons. American Journal of Tropical Medicine and Hygiene 23: 974-982.
- Halstead SB, Nimmannitya S, Cohen SN (1970) Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale Journal of Biology and Medicine 42: 311-328.
- Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, et al. (1984) Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. American Journal of Epidemiology 120: 653-669.
- Guzman MG, Kouri G, Valdes L, Bravo J, Alvarez M, et al. (2000) Epidemiologic studies on dengue in Santiago de Cuba, 1997. American Journal of Epidemiology 152: 793-799.
- Halstead SB (1993) Pathophysiology and pathogenesis of dengue haemorrhagic fever. In: Thongchareon P, ed. Monograph on dengue/dengue haemorrhagic fever. New Delhi, World Health Organization, Regional Office for South-East Asia 1993: 80-103.
- Halstead SB (1989) Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Reviews of Infectious Diseases 11: S830-S839.
- Halstead SB, Heinz FX, Barrett AD, Roehrig JT (2005) Dengue virus: molecular basis of cell entry and pathogenesis, 25-27 June 2003, Vienna, Austria. Vaccine 23: 849-856.
- Kouri GP, Guzman MG, Bravo JR, Triana C (1989) Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bulletin of the World Health Organization 67: 375-380.
- De la C Sierra B, Kouri G, Guzman MG (2007) Race: a risk factor for dengue hemorrhagic fever. Archives of Virology 152: 533-542.
- Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M (1998) Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. Journal of Immunology 161: 6338-6346.
- Cardier JE, Mariño E, Romano E, Taylor P, Liprandi F, et al. (2005) Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: possible role of TNF-alpha in endothelial cell damage in dengue. Cytokine 30: 359-365.
- Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, et al. (1998) Dengue and dengue haemorrhagic fever. Lancet 352: 971-977.
- Yip WCL. Dengue haemorrhagic fever: current approaches to management. Medical Progress, October 1980.
- Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, et al. (1997) Early clinical and laboratory indicators of acute dengue illness. Journal of Infectious Diseases 176: 313-321.
- Cao XT, Ngo TN, Wills B, Kneen R, Nguyen TT, et al. (2005) Evaluation of the World Health Organization standard tourniquet test in the diagnosis of dengue infection in Vietnam. Tropical Medicine and International Health 7: 125-132.
- Balmaseda A, Hammond SN, Pérez MA, Cuadra R, Solano S, et al. (2005) Assessment of the World Health Organization scheme for classification of dengue severity in Nicaragua. American Journal of Tropical Medicine and Hygiene 73: 1059-1062.
- Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, Wongtapradit L, Nithipanya N, et al. (2007) Natural history of plasma leakage in dengue hemorrhagic fever: a serial ultrasonic study. Pediatric Infectious Disease Journal 26: 283-290.
- Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR (1969) Dengue and chikungunya virus infection in man in Thailand, 1962–64. Observations on hospitalized patients with haemorrhagic fever. American Journal of Tropical Medicine and Hygiene 18: 954-971.
- Martinez-Torres E, Polanco-Anaya AC, Pleites-Sandoval EB (2008) Why and how children with dengue die? Revista cubana de medicina tropical 60: 40-47.
- Nimmannitya S (1987) Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian Journal of Tropical Medicine and Public Health 18: 392-397.
